Clinicians have powerful tools to combat STIs: effective antibiotics, mobile testing units, and they can easily order STI testing for high-risk patients during routine visits. Yet STI rates continue to persist at crisis levels. Why? Perhaps it is because there is a weak link in their clinical workflow that can diminish the impact of all their tools: diagnostic testing delays. When STI lab tests are “sent out” patients must wait days for results and up to 60% of those patients can be lost-to-follow-up.1 But this seemingly intractable problem has a surprisingly simple solution.
A wait-and-see process isn’t meeting an urgent here-and-now need for STI Testing
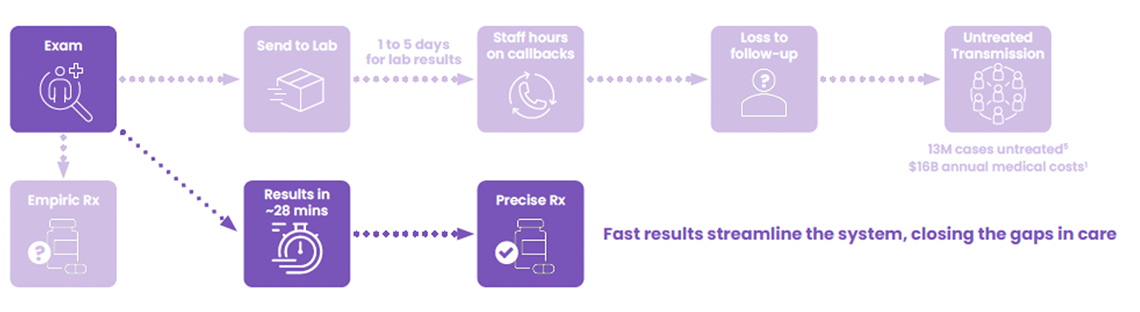
Today’s STI testing creates a cruel paradox: the more accurate the test, the longer patients wait—and the more likely they are to disappear. With 90% of STI diagnostic tests sent to external labs for PCR analysis, patients face 3-5 day delays for results.2 During this gap, 20-60% of patients are lost to follow-up,2 never receiving treatment even when infections are confirmed or receiving the wrong treatment because the clinician is forced to treat empirically knowing the likelihood of their patient being lost-to-follow-up.
This loss occurs precisely when STIs are surging nationwide. The CDC reported over 2.4 million cases of chlamydia, gonorrhea, and syphilis in 2023 alone,3,4 with trichomoniasis adding another 3.7 million cases—more than chlamydia and gonorrhea combined.3,4 Most troubling: half of new STI infections occur among young people aged 15-24, highlighting the vulnerability of the younger population.5
So, despite all our clinical and community intervention efforts, even when tested and diagnosed, too many STI patients are lost to follow-up because of this diagnostic delay.2
The high cost of delayed STI diagnostic results
There are many consequences associated with diagnostic delays across the continuum of care.
- Patient Impact: Diagnostic delays create treatment anxiety and avoidance behaviors. Patients facing days of uncertainty often skip follow-up appointments entirely, leaving infections untreated and fueling transmission.
- Clinical Impact: Anticipating patient loss, antibiotics are often prescribed without a confirmed diagnosis. This can result in inaccurate or overtreatment and increase the chances of antibiotic resistance.1 Continued empiric treatment practices can result in hospitalizations and more advanced care, costing $4.6 billion annually. 1,7
- Operational Impact: Send-out testing drains resources through processing, transporting, and ineffective callback procedures. Staff spend valuable time tracking down patients instead of delivering care—a critical inefficiency when healthcare facilities already struggle with recruiting, retaining or simply paying their staff.
- System-Wide Impact: Untreated STIs cost the healthcare system $16 billion annually in complications and advanced care.6 Every delayed diagnosis multiplies these costs while accelerating community transmission.
From Days to Minutes: Point-of-Care PCR STI Testing Changes Everything
The Visby Sexual Health Test transforms the fundamental equation of STI care. Instead of days, results arrive in 28 minutes. Instead of callbacks and lost patients, treatment happens in the same visit. Instead of treating empirically, clinicians get PCR reliable results while the patient waits, so they can treat precisely and confidently.
This isn’t just faster testing, it’s a complete workflow transformation. Mobile units can now provide complete care on-site. Busy clinics can resolve STI cases immediately, freeing resources for patient care. High-risk patients being seen for other conditions can now be tested and treated without additional visits. One visit, one swab, one clear answer. It’s not just faster care — it’s decisive care, delivered when the patient is still in the room.8
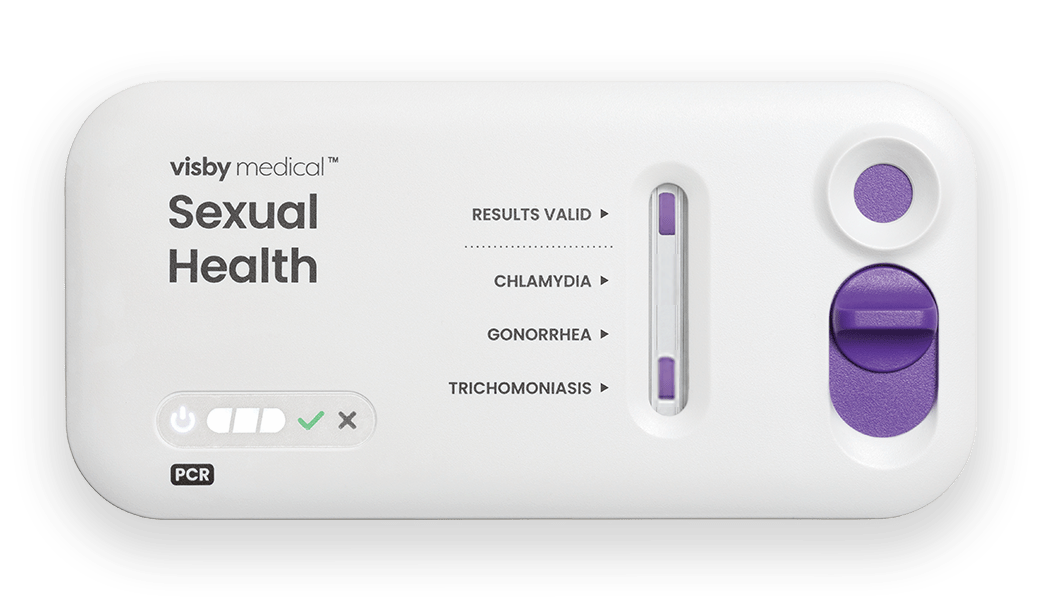
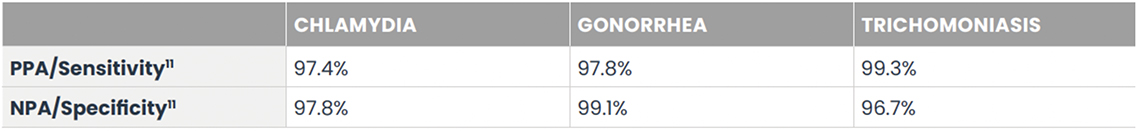
The Visby Sexual Health Test is the only CLIA-waived PCR STI test to provide results for the 3 most common and treatable STIs: chlamydia, gonorrhea, and trichomoniasis. Delivering PCR results right there and then with the patient present, this device can untether facilities from central lab send-outs and complex and costly instrumentation. Fitting in the palm of a clinician’s hand, the device only needs one vaginal sample swab for all three tests.8
Transforming wait-and-see to right, here and now
Right: PCR results, cleared for asymptomatic and symptomatic individuals8
Here: Portable, no instrument or refrigeration required8
Now: Results in ~28 minutes8
A diagnostic innovation that fixes the weakest link in STI care
The weakest link in STI care isn’t lack of treatments or clinical expertise, it’s time. Every day of diagnostic delay because of sending out STI testing creates another opportunity for patients to slip away and infections to spread. The Visby Sexual Health Test eliminates that delay, transforming STI care from reactive to timely and precise. The question isn’t whether point-of-care testing will revolutionize STI management – it’s how quickly healthcare providers will adopt this game-changing technology.
References
- Garlock J, Lee L, Cucci M, Frazee LA, Mullen C. Suspected gonorrhea and chlamydia: Incidence and utilization of empiric antibiotics in a health system emergency department. Am J Emerg Med. 2019;37(5):884-889. doi: 10.1016/j.ajem.2018.08.015
- Huppert JS, Reed JL, Munafo JK, et al. Improving notification of sexually transmitted infections: a quality improvement project and planned experiment. Pediatrics. 2012 Aug;130(2):e415-22. doi: 10.1542/peds.2011-3326
- Sexually Transmitted Infection Statistics. National Overview of STIs in 2023. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti-statistics/annual/summary.html
- Meites E. Trichomoniasis: the “neglected” sexually transmitted disease. Infect Dis Clin North Am. 2013;27(4):755-64. doi:10.1016/j.idc.2013.06.003
- Sexually Transmitted Infection Statistics. Sexually transmitted infections surveillance, 2023. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti-statistics/annual/index.html
- Sexually Transmitted Infections. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti/php/communication-resources/prevalence-incidence-and-cost-estimates.html
- Centers for Disease Control and Prevention. CDC partners estimate healthcare cost of antimicrobial resistant infections. CDC website. Published February 4, 2025. Accessed June 11, 2025. https://www.cdc.gov/antimicrobial-resistance/stories/partner-estimates.html
- Visby Medical Sexual Health Test. Instructions for Use. 2025. Visby Medical.
- Sexually Transmitted Infection Statistics. National Overview of STIs in 2023. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti-statistics/annual/summary.html
- Meites E. Trichomoniasis: the “neglected” sexually transmitted disease. Infect Dis Clin North Am. 2013;27(4):755-64. doi:10.1016/j.idc.2013.06.003
- Sexually Transmitted Infections Treatment Guidelines, 2021. Trichomoniasis. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
- Meites E, Llata E, Braxton J, et al. Trichomonas vaginalis in selected U.S. sexually transmitted disease clinics: testing, screening, and prevalence. Sex Transm Dis. 2013 Nov;40(11):865-9. doi: 10.1097/OLQ.0000000000000038
