
Visby Medical™ Raises up to $65 Million in New Financing Round to Accelerate Launch of Groundbreaking At-Home Sexually Transmitted Infections (STI) Test
Visby Medical secures up to $65 million in funding led by Catalio Capital Management to accelerate the commercial launch of their FDA-authorized at-home STI test for women. The revolutionary PCR diagnostic delivers lab-accurate results in 30 minutes via smartphone app and will be available starting July 2025.

Visby Medical Submits Men‘s Sexual Health Test to FDA for Clearance and CLIA Waiver
Visby Medical has submitted its Men’s Sexual Health Test to the FDA for clearance and CLIA waiver, which would provide PCR results for Chlamydia and Gonorrhea detection from male urine samples in under 30 minutes if cleared. The single-use, instrument-free point-of-care test requires no capital investment or maintenance and can be easily integrated into diverse clinical settings including urgent care centers and physician offices. This submission expands Visby Medical’s sexual health diagnostic portfolio and helps address the critical need for streamlined STI testing.

Visby Medical™ Receives Landmark FDA De Novo Authorization for First-Ever OTC PCR Test for Sexual Health
Visby Medical™ secured FDA De Novo authorization for the first OTC PCR sexual health test for women, marking a significant market entry into at-home diagnostics. The innovative, palm-sized device enables rapid, reliable testing for three common sexually transmitted infections, addressing a substantial unmet medical need. With plans for strategic partnerships and expansion into additional diagnostic markets, the company positions itself to capitalize on the growing demand for convenient, private healthcare solutions.

Visby Medical™ Receives FDA Clearance and CLIA Waiver for Point-of-Care Respiratory Health Test
The Visby Medical platform offers true PCR technology; PCR is the gold standard for testing Flu A, Flu B, and COVID-19. The Visby Medical Respiratory Health Test, which fits in the palm of your hand, provides accurate results in under 30 minutes at the point of care, enabling clinicians to accurately diagnose and treat patients even in remote care facilities and other resource-limited healthcare settings where centralized laboratory services are less accessible.

Visby Medical Secures Additional $3.9M from CARB-X to Fight Antibiotic Resistance with PCR-Based Mutation Detection at the POC
Point-of-Care test significantly shortens time from ED arrival to test results, treatment and discharge – significant improvements are seen in the use of antibiotics for the treatment of chlamydia and gonococcal infections in women. Nationwide increases in sexually transmitted diseases and antibiotic resistance create the need for a paradigm shift from traditional lab-based molecular testing.

Visby Medical™ Sexual Health Test Results in More Appropriate Antibiotic Treatment and Shorter Emergency Department Visits Than Standard of Care
Point-of-Care test significantly shortens time from ED arrival to test results, treatment and discharge – significant improvements are seen in the use of antibiotics for the treatment of chlamydia and gonococcal infections in women. Nationwide increases in sexually transmitted diseases and antibiotic resistance create the need for a paradigm shift from traditional lab-based molecular testing.
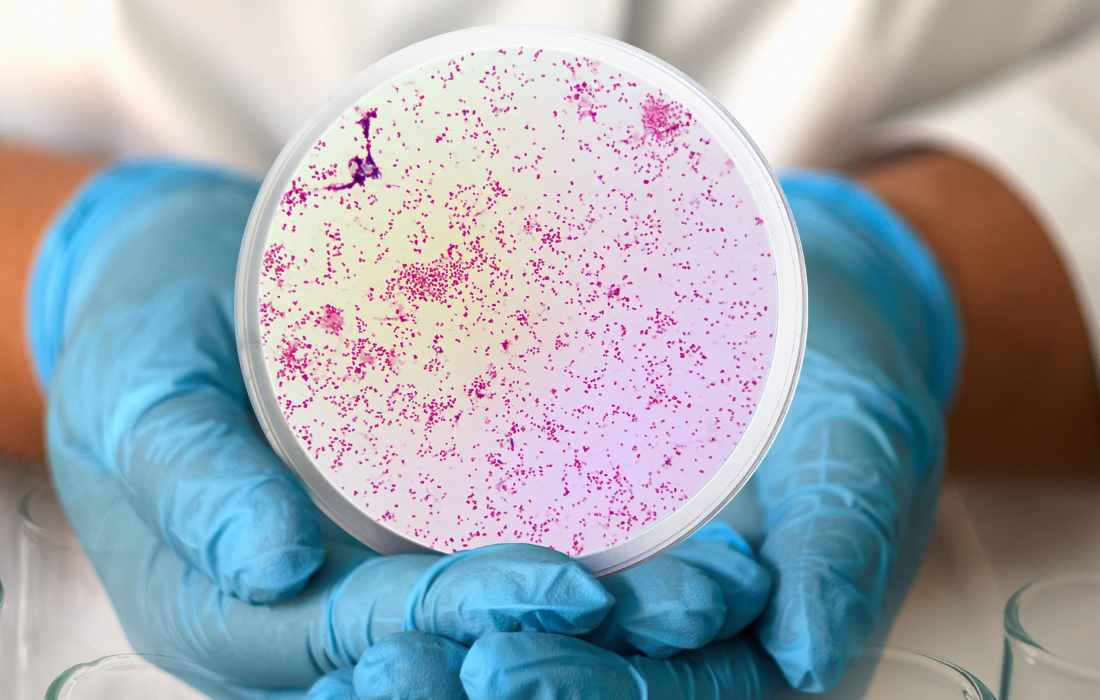
CARB-X awards funding to Visby Medical for gonorrhea, antibiotic susceptibility test
The Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) announced today that it will award biotechnology company Visby Medical of San Jose, California, up to $1.8 million to develop a portable polymerase chain reaction (PCR) test that can detect gonorrhea and assess its susceptibility to ciprofloxacin.

Visby Medical™ Receives FDA Clearance and CLIA Waiver for second Generation Sexual Health Test for Women
Visby Medical™ announced today that it has received 510(k) clearance and was granted a CLIA waiver from the FDA for its 2nd generation point of care (POC) test.

Visby Medical Receives FDA Emergency Use Authorization for Respiratory Health Test for Use in CLIA Waived Settings
Visby Medical announced today that it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration for its Respiratory Health point of care test.

Visby Medical Expands Series E Round to Over $135 Million
Visby Medical extends Series E round to include an additional $35 million, totaling more than $135 million raised.

Visby Reaffirms Support for Women’s Access to Healthcare
Visby Medical’s CEO reaffirms support for women’s healthcare access, announcing travel expense reimbursement for employees for out-of-state pregnancy-related care.

AACC selects Visby Medical as 2022 Disruptive Technology Award Finalist
AACC selects Visby Medical as a finalist for the 2022 Disruptive Technology Award for its handheld point-of-care PCR diagnostic technology.

Visby Medical Appoints Everett Cunningham to Board of Directors
Visby Medical today announced Everett Cunningham has been appointed to the company’s Board of Directors.

Chiricahua Community Health Centers, Inc., Launches STI Testing Program for Women with Same-Day Results
The Body Agency Collective, Visby Medical, Inc. and CCHCI observe National STD Awareness Month with women’s health initiative on US-Mexico border.
CCHCI among first healthcare providers in the nation to adopt new handheld PCR technology to detect gonorrhea, chlamydia and trichomoniasis in a single visit.

Urgent Care For Children Collaborates With Visby Medical To Provide New Gold Standard Diagnostic For Sexually Transmitted Infections
Pediatric urgent care provider among first in the US to adopt new point-of-care STI diagnostic device in adolescents and young adults

Visby Medical Executes Contract Option with BARDA for $25.5M to Develop Rapid Flu-COVID PCR Test Designed for At-Home Use
Visby Medical today announced that it has received an additional $25.5M in federal funding to develop and validate an at-home combination Flu-Covid test from the Biomedical Advanced Research and Development Authority (BARDA)

Visby Medical Raises over $100 Million in Series E Financing
Investment will accelerate the development of revolutionary rapid, single-use PCR diagnostic technology for home use

Visby Medical to Present at the J.P. Morgan 40th Annual Healthcare Conference
Visby Medical™ announced that it will make its debut appearance at the 40th Annual J.P. Morgan Healthcare Conference.

Visby Medical Appoints Terri S. Bresenham and Scott Edward Mendel to Board of Directors
Visby Medical™ today announced Terri S. Bresenham and Scott Edward Mendel have been appointed to the company’s Board of Directors.

Clinical Data of The Visby Medical Sexual Health Click Test Published in Journal of the American Sexually Transmitted Diseases Association
Integration of the Visby Medical sexually transmitted infections (STI) panel into clinical practice could significantly reduce over- and under-treatment of Chlamydia, Gonorrhea, and Trichomonas

Visby Medical Appoints Industry Veteran Thomas Prescott as Chairman of the Board
Thomas Prescott, former president and chief executive officer (CEO) of Align Technology and Cardiac Pathways, will join as the company’s new chairman of the board.

Visby Medical Appoints Industry Veteran John W. Kuo as Chief Legal Officer
Visby Medical™, a leading medical diagnostic company, today announced the appointment of John W. Kuo as the company’s new Chief Legal Officer. An experienced veteran in corporate law, Kuo brings more than 30 years of experience across multiple industries, including nearly 20 years in the healthcare industry.
FDA Grants Emergency Use Authorization for Visby Medical’s COVID-19 RT-PCR Test for Pooled Samples
The Visby Medical™ instrument-free Reverse Transcription (RT)-Polymerase Chain Reaction (PCR) COVID-19 test was granted Emergency Use Authorization (EUA) by the FDA

Visby Medical™ Receives FDA Clearance and CLIA Waiver at the Point of Care for PCR Sexual Health Test
The Visby Medical Sexual Health Click Test is the first instrument-free PCR test for the detection of Chlamydia, Gonorrhea, and Trichomonas, with results available within 30 minutes, during the patient visit.

Visby Medical’s COVID-19 PCR Point of Care Test Authorized for Use in CLIA Waived Settings
Expanding critical access to the only single-use, rapid PCR test, at the point of care.

Visby Medical speeds development of a rapid Flu-COVID PCR test designed for at-home use with BARDA funding
Visby Medical pairs speed with the accuracy of gold-standard PCR testing in a single-use device, so doctors don’t have to fight blind.
Visby Medical’s Personal PCR Receives Funding from National Institute of Health, RADx Program
Visby Medical, an infectious disease diagnostic company, received funding from the National Institutes of Health as part of the organization’s Rapid Acceleration of Diagnostics (RADx) program, an initiative that aims to combat SARS-CoV-2 and increase national testing capacity for COVID-19.

Visby Medical’s Personal PCR Device Receives FDA Emergency Use Authorization for Moderate-Complexity Lab Environments
Seven-year-old startup emerges from stealth mode, provides adaptable and rapid testing technology to frontline lab personnel fighting the pandemic
Rapid diagnostic for gonorrhea wins $19 million federal prize competition to combat antibiotic resistance
As appeared in NIH website 8/5/20