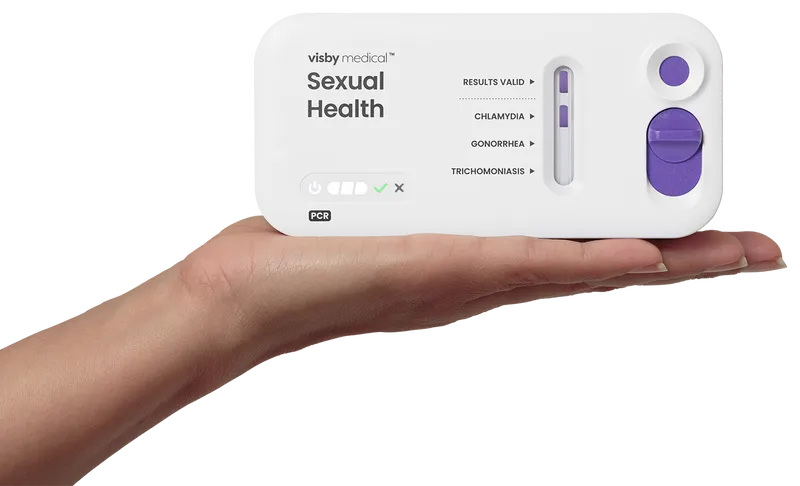
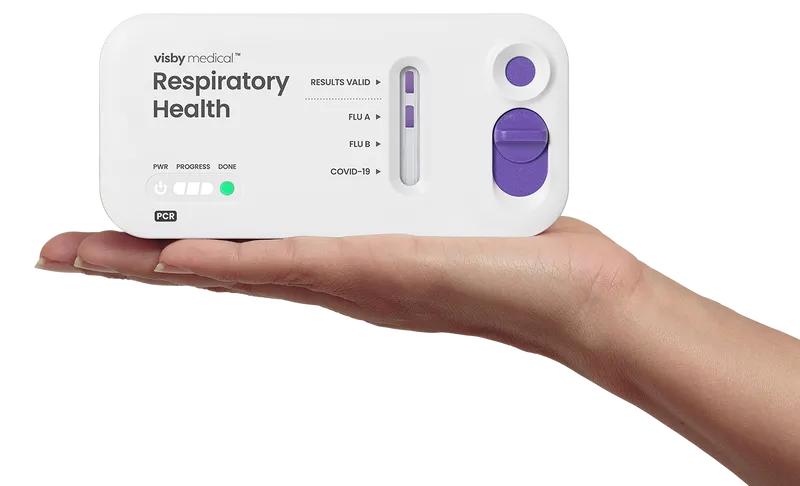
The power of PCR
in your hands
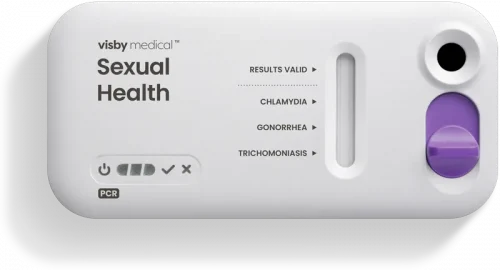
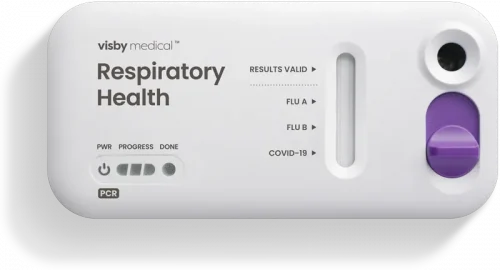
Results delivered at the point of care in under 30 minutes
The Only Instrument-Free,
Rapid PCR Test
Our rapid PCR tests provide accurate and reliable results for healthcare providers all across the United States. We are a medical diagnostics company with portable PCR testing technology that may be implemented in both clinical and mobile laboratory settings.
Visby Medical strives to make access to infectious disease testing as easy and as accessible as possible.

PCR results
in under 30 minutes

Portable, deployable, and scalable

PCR accuracy

No instrument,
no maintenance

No capital investment, no service contracts
PCR Results in
Less Than 30 Minutes
In a world where speed and accuracy in disease testing has become increasingly important, the efficient protection of public health depends on the delivery of fast and reliable infectious disease testing to affected communities as quickly as possible.
The Visby Medical portable, instrument-free PCR test delivers results in minutes, not days, making our PCR test kits a revolution in the molecular diagnostics industry.
The following are five key benefits to using Visby PCR in your lab or clinic, right at the point of care.
What is Visby PCR?
Medical Company
with a Human Focus
Our aim is to continue developing testing devices that are reliably accurate. The current lack of rapid PCR testing available to inform a timely and accurate diagnosis for the patient demonstrates the inherent need for the Visby Medical diagnostic platform.
PCR results to treat in the same visit. That’s a first!
Visby Medical reduced a ~6-day1 central lab wait time down to under 30 minutes at the point-of-care. You can walk into the exam room with the answer, patients walk out with confidence. Patient callbacks and loss to follow up may become a thing of the past.
The biggest improvements are often the smallest.
Visby Medical shrank the traditional sofa-sized PCR lab-instrument down to the palm of your hand. Now it’s portable, and easily deployable, in a 5″ by 2″ device. Revolutionary.
Small and mighty fast.
Surge capacity, without capital investment or maintenance.
Recognition:
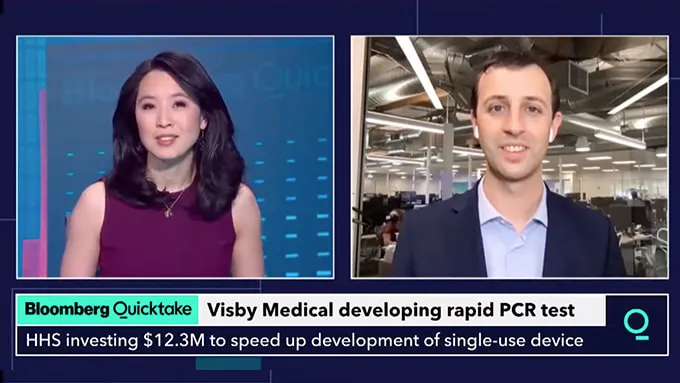
February 2, 2021: Visby receives a $12.3 million award from BARDA to speed development of its rapid, single-use Flu-COVID PCR Test.
Read more

November 23, 2020: Visby Medical Sexual Health Test clinical study, sponsored by NIH/NIAID, is published in The Lancet: “Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study.”
Read more

October 6, 2020: Visby receives $10 Million from the NIH as part of its Rapid Acceleration of Diagnostics (RADx) initiative.
Read more

August 5, 2020: Visby Medical selected as the ONLY winner of $19 Million in the Antimicrobial Resistance (AMR) Diagnostic Challenge (co-sponsored by BARDA and the NIH).
Read more

Are you ready to bring the power of lab PCR
to your point of care clinic?