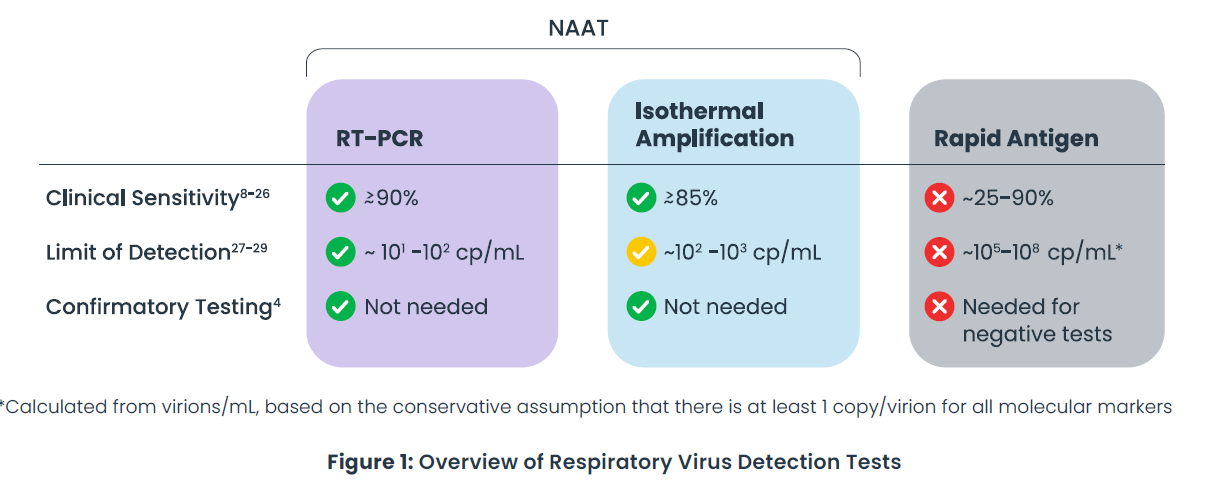
Accurate and timely diagnosis of respiratory infections is critical to guide appropriate treatment and limit unnecessary use of antibiotics. This white paper, Superiority of RT-PCR Tests for the Detection of Respiratory Viruses at the Point-of-Care, summarizes clinical performance data from over a dozen peer-reviewed publications and product validations. It compares rapid antigen tests, isothermal nucleic acid amplification tests (NAATs), and RT-PCR tests for detecting influenza A, influenza B, and SARS-CoV-2.
The findings show that RT-PCR tests provide the highest sensitivity (95–100%), followed by isothermal NAATs (88–97%), with rapid antigen tests trailing significantly (25–90%). While antigen tests are fast and inexpensive, they risk false negatives and may require confirmatory testing. In contrast, newer point-of-care RT-PCR platforms offer both speed and exceptional accuracy—without the delays of send-out lab testing.
The Visby Medical Respiratory Health Test stands out as the only instrument-free RT-PCR option, combining clinical accuracy with a compact, self-contained format that is ideal for urgent care, outpatient clinics, and other decentralized settings.