The US health system is confronting an escalating crisis in sexual health: sexually transmitted infections (STIs) continue surging while federal funding for community health services faces dramatic cuts. Community and public health facilities have long struggled to manage STI transmission with limited resources, but now they face the compounding challenge of reduced federal support they depend on.1 With overwhelming need and diminishing resources, providers are being asked to achieve the impossible in protecting vulnerable communities. However, an innovative solution emerges through alternative diagnostic technology that can transform STI care into a more efficient and precise process.
An Escalating STI Crisis Worsened by Federal Cuts
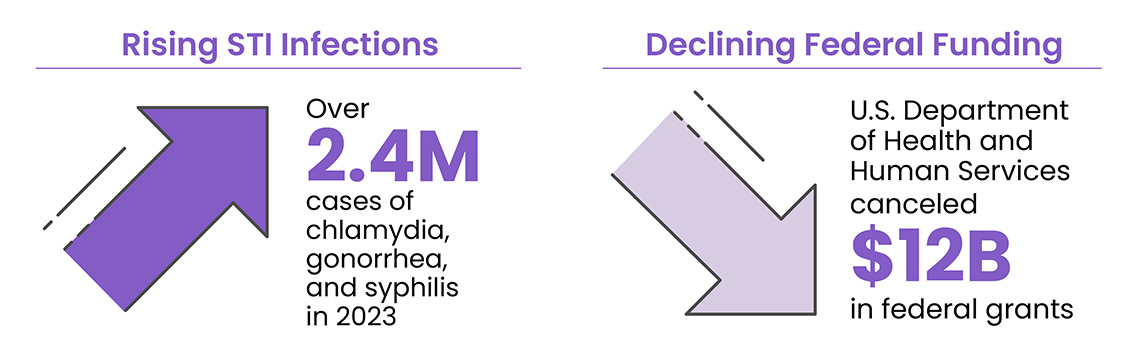
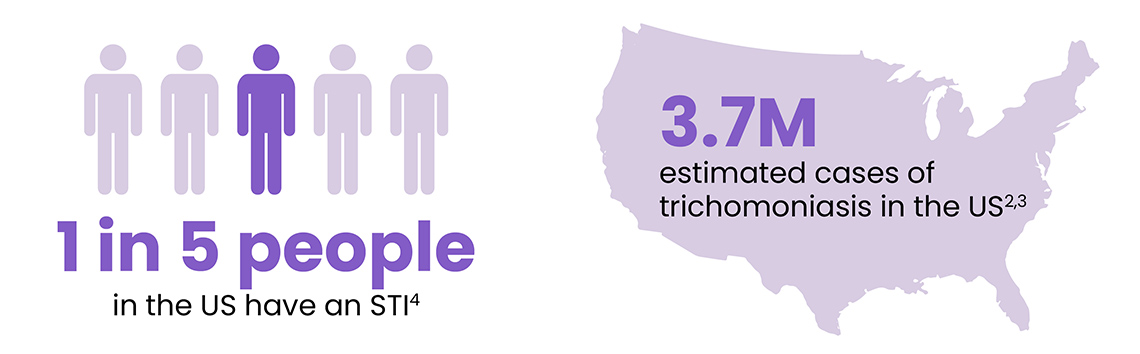
Despite being easily treated and cured, STIs are reaching alarming levels across the U.S. The Centers for Disease Control and Prevention (CDC) reported over 2.4 million cases of chlamydia, gonorrhea, and syphilis in 2023 alone. Chlamydia led with over 1.6 million cases, while gonorrhea and syphilis contributed over 600,000 and 209,000 cases, respectively.2,3 Perhaps most concerning, trichomoniasis—which is highly asymptomatic and under-reported—accounts for more US cases than chlamydia and gonorrhea combined, affecting approximately 3.7 million Americans annually.2,3 The vulnerability of younger populations becomes evident when considering that approximately 50% of new STI cases occur among individuals aged 15-24.4
The crisis deepened in early 2025 when the U.S. Department of Health and Human Services canceled approximately $12 billion in federal grants originally allocated during the COVID-19 pandemic, significantly impacting infectious disease and mental health programs.5 These substantial cuts have forced many community health organizations to reduce services, lay off essential staff, or close entirely, creating a perfect storm that exacerbates the challenges in managing the STI epidemic.
The Hidden Costs of Traditional Diagnostic Workflows
One of the biggest challenges I see today is balancing the pace of innovation with real-world adoption. We’re seeing incredible advances in molecular diagnostics, digital health integration, and point-of-care solutions, but health systems often struggle to absorb these innovations due to entrenched workflows, reimbursement barriers, and a patchwork of regulatory expectations—especially in decentralized or at-home settings. Reimagining traditional STI diagnostic testing workflows could be just the game-changer needed. Traditional STI diagnostic processes of sending out lab tests create delays in results and therefore treatment that can strain resources and create critical gaps in care delivery.
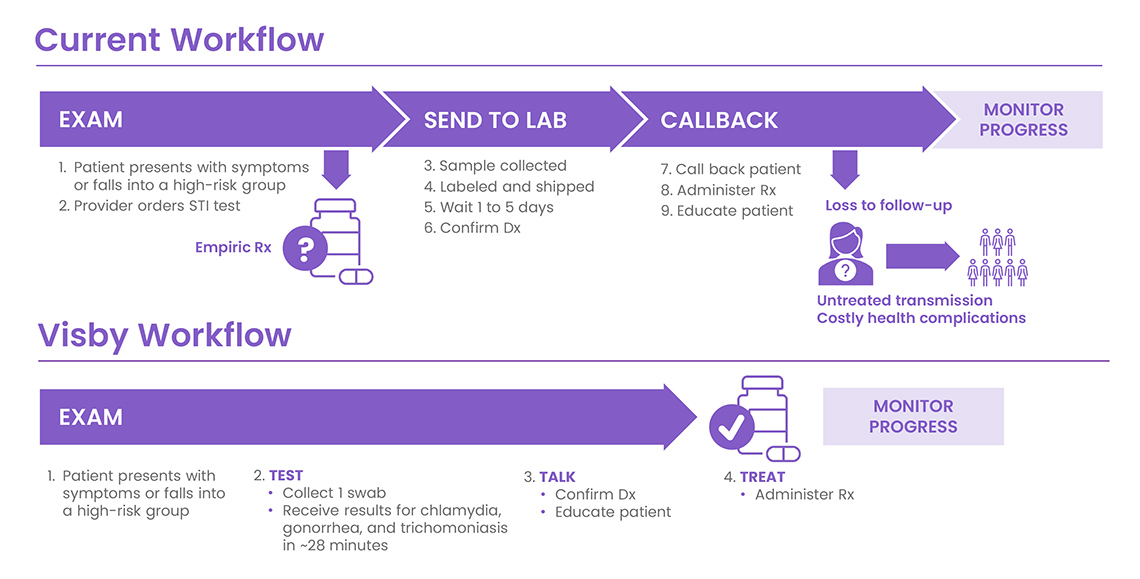
Currently, 90% of STI diagnostic tests rely on external laboratories for nucleic acid amplification testing (NAAT), commonly known as PCR tests. While these represent the gold standard for the precise results needed for effective treatment, the accuracy comes at a cost: 3 to 5 days of wait time. This delay triggers a cascade of costly clinical and administrative burdens that overwhelmed facilities can no longer afford. There are also consequences for patients. When patients wait days for results, studies show that 20% to 60% never return for their results or subsequent treatment.6 This gap in care doesn’t just affect individual health outcomes, it can result in more costly health complications later while continuing to perpetuate community transmission.7
From an operational perspective, reliance on centralized labs with extended turnaround times forces facilities to dedicate precious staff hours to callbacks—the process of contacting patients, communicating test results, and arranging treatment. With staff cuts and shrinking resources, facilities increasingly struggle to divert time and energy to these administrative tasks that could be better spent on direct patient care.
Innovating Out of a Funding Crisis: The Point-of-Care Solution
The healthcare industry now has an opportunity to overcome these costly challenges through rapid, point-of-care PCR STI testing. Visby Medical has developed the Sexual Health Test—a portable, single-use diagnostic device that delivers results in approximately 28 minutes.8 The Visby Sexual Health Test is the only CLIA-waived PCR STI test capable of providing results for the three most common and treatable STIs: chlamydia, gonorrhea, and trichomoniasis.8
This compact, rapid PCR testing solution eliminates costly send-out laboratory dependencies and frees overburdened staff to focus on patient care rather than administrative callbacks. It represents a smarter, leaner approach to STI management—one that safeguards both budgets and patient outcomes.
By delivering PCR-quality results immediately with the patient present, this device liberates facilities from central lab send-outs and complex, costly instrumentation. Fitting in the palm of a clinician’s hand, the device requires only one vaginal sample swab for all three tests.8 With immediate and accurate results, clinicians can treat decisively and precisely, avoiding the less-precise empirical antibiotic treatment commonly practiced with patient populations who frequently don’t return for follow-up care.9
The benefits extend beyond clinical efficiency. Patients can also experience relief by resolving their condition in a single visit instead of enduring anxious days awaiting results. Meanwhile, healthcare facilities gain better control over community transmission without requiring additional staff or resources during these challenging times.
A Strategic Path Forward: Rapid PCR Testing
With STI rates continuing to rise and federal support diminishing, healthcare organizations must identify innovative approaches to maintain quality care despite budget constraints. A strategic reevaluation of traditional diagnostic workflows presents an immediately actionable solution. Implementing rapid PCR testing in community and public health settings can help mitigate the impact of funding cuts, maintain quality care standards, and strengthen efforts to control STI transmission in the communities that need it most.
The dual threat of rising STI rates and reduced federal funding demands creative solutions that work within new financial realities. Point-of-care diagnostic technology offers a pathway to transform these challenges into opportunities for more efficient, effective care delivery.
References
- STD Awareness Month 2018: Impact of Budget Cuts on HIV, STI, and Viral Hepatitis Programs. National Association of County Health Officials website. Accessed June 25, 2025. https://www.naccho.org/blog/articles/std-awareness-month-2018-impact-of-budget-cuts-on-hiv-sti-and-viral-hepatitis-programs
- Sexually Transmitted Infection Statistics. National Overview of STIs in 2023. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti-statistics/annual/summary.html
- Meites E. Trichomoniasis: the “neglected” sexually transmitted disease. Infect Dis Clin North Am. 2013;27(4):755-64. doi:10.1016/j.idc.2013.06.003
- Sexually Transmitted Infection Statistics. Sexually transmitted infections surveillance, 2023. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti-statistics/annual/index.html
- US pulls back $12 billion in funding to state health departments. Reuters website. https://www.reuters.com/business/healthcare-pharmaceuticals/us-government-pulls-back-over-11-billion-funding-state-health-departments-2025-03-26/
- Huppert JS, Reed JL, Munafo JK, et al. Improving notification of sexually transmitted infections: a quality improvement project and planned experiment. Pediatrics. 2012 Aug;130(2):e415-22. doi: 10.1542/peds.2011-3326
- Sexually Transmitted Infections. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. Centers for Disease Control and Prevention website. Accessed April 2, 2025. https://www.cdc.gov/sti/php/communication-resources/prevalence-incidence-and-cost-estimates.html
- Visby Medical Sexual Health Test. Instructions for Use. 2025. Visby Medical.
- Garlock J, Lee L, Cucci M, Frazee LA, Mullen C. Suspected gonorrhea and chlamydia: Incidence and utilization of empiric antibiotics in a health system emergency department. Am J Emerg Med. 2019;37(5):884-889. doi: 10.1016/j.ajem.2018.08.015
- Centers for Disease Control and Prevention. CDC partners estimate healthcare cost of antimicrobial resistant infections. CDC website. Published February 4, 2025. Accessed June 11, 2025. https://www.cdc.gov/antimicrobial-resistance/stories/partner-estimates.html
